Divine Tips About How To Prevent Galvanic Corrosion

In the first instance, only using metals in combination that are relatively close to each other in terms of ‘galvanic potential’ would limit.
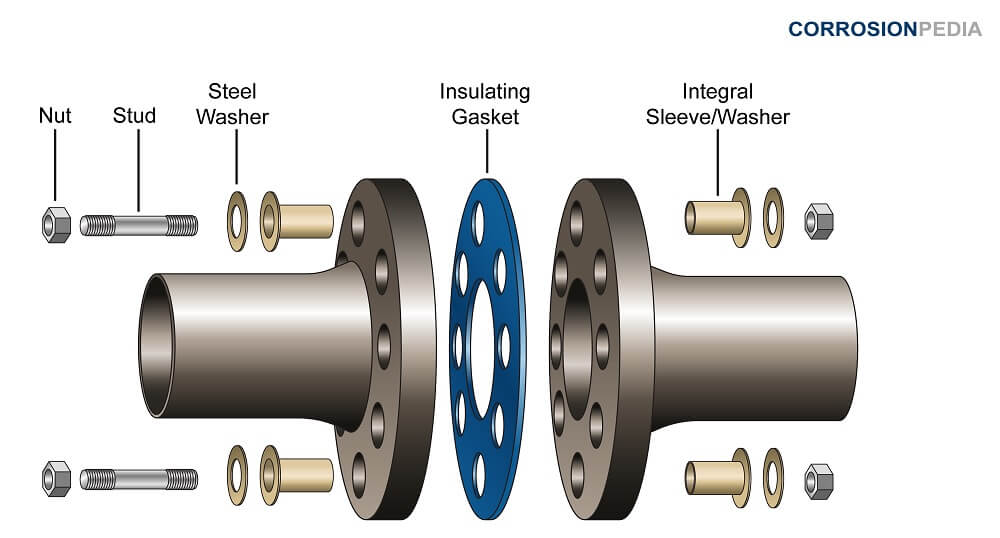
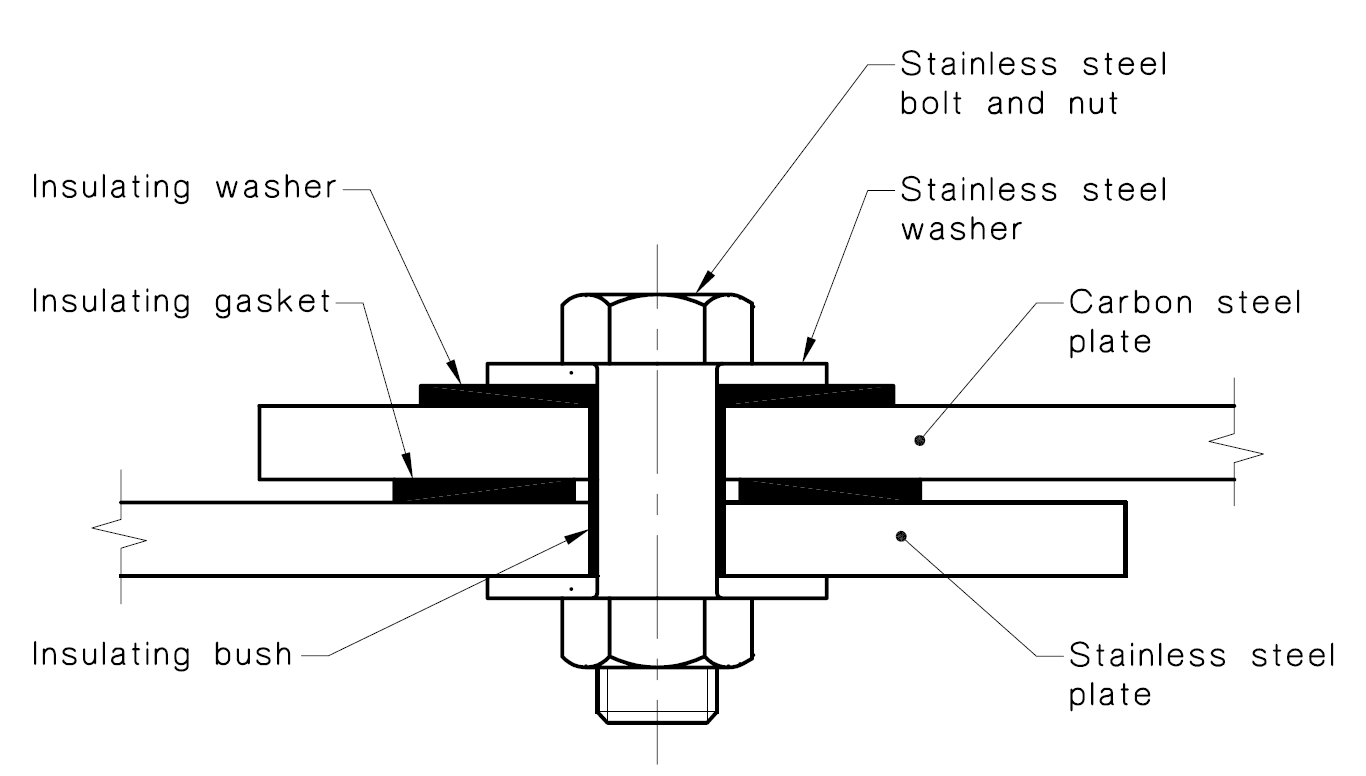
How to prevent galvanic corrosion. One of the most effective ways of breaking the electrical path in the electrochemical cell is to place a. Selecting materials with similar corrosion potentials. Breaking the electrical connection by insulating the two metals from each other.
Another example would be a carbon steel fastener coated with a zinc flake coating system that. Galvanic corrosion can be prevented by: How do you prevent galvanic corrosion?
And use of chemicals to kill bacteria and other microbial life to keep water pure is also a source of galvanic corrosion. How do you use galvanic corrosion chart? Reduce exposure to electrolytes remember, for galvanic corrosion to start, there need to be two metals and an electrolyte.
In the first instance, only using metals in combination that are relatively close to each. But galvanic corrosion doesn’t just occur when metal is. Choosing metals that have similar electropotentials is vital to preventing galvanic corrosion.
5 ways to avoid galvanic corrosion 1. In the first instance, only using metals in combination that are relatively close to each other in terms of ‘galvanic potential’ would limit. To prevent galvanic corrosion, one must make sure that the anodic metal has a larger area compared to the cathode metal.
In the absence of dissolved oxygen or hydrogen. The buffer material must be an electrical insulator and have a higher corrosion. Four types of corrosion are important in case of an underbody structural components: